Our Research
We take advantage of various imaging modalities, including structural and functional imaging of Primary Progressive Aphasia (PPA). In collaboration with the Memory and Aging Center (MAC) Imaging Core and the Rab Lab, we assist in the development of various brain imaging pipelines, which we utilize alongside behavioral and cognitive assessments. Currently, our structural magnetic resonance imaging (sMRI) protocol comprises T1- and T2-weighted imaging for gray matter structure and diffusion-weighted imaging (DWI) for white matter structure. We analyze functional imaging data acquired using functional magnetic resonance imaging (fMRI), magnetoencephalography (MEG), and positron emission tomography (PET). Together, these imaging modalities allow us to conduct a thorough investigation of the clinicopathological correlates of PPA from different, but complementary, angles.
Our Areas of Interest
• Neuroimaging (sMRI, fMRI, DTI, MEG, PET) of PPA clinical and pathological correlates
• Distributed semantics via novel behavioral assessments and MEG studies
• Prediction of longitudinal gray matter changes in PPA based on pre-defined healthy functional networks
• Deep learning and advanced mathematical models in PPA for prediction of in-vivo pathological changes in PPA
• Longitudinal tracking of structural changes in PPA and its association with behavioral and cognitive impairment
• Multilingualism-related differences in clinical presentation and neural correlates of PPA
• Theory- and data-driven clustering of PPA into reproducible clinical syndromes and associated brain atrophy patterns
• Dissection of white matter pathways related to language and speech features in PPA
• Intrinsic connectivity networks critical for specific aspects of language in PPA
• Machine learning predictive modeling of cognitive and language decline in PPA
• Behaviorally relevant compensatory neural mechanisms in PPA
• Treatment-induced structural and functional neuroplasticity in PPA
• Structural and functional organization of language in the human brain
sMRI: Neural Correlates in PPA

Spatial distribution of the four task-free networks. Rendered left-hemisphere lateral views of the four ICNs identified through a statistical conjunction of the two groups. Maps were thresholded at the same statistical threshold used to identify the networks for each group separately, binarized and colored using the same color scheme in Figure 1 (blue for the network seeded from the pITG, yellow for the network seeded from the Spt, red for the network seeded from the opIFG, and green for the one seeded from the aMTG). The render at the center of the figure shows that the four networks cover almost the entire left hemisphere with a small degree of overlap.
Battistella G, Borghesani V, Henry M, Shwe W, Lauricella M, Miller Z, Deleon J, Miller BL, Dronkers N, Brambati SM, Seeley WW, Mandelli ML, Gorno-Tempini ML. Task-Free Functional Language Networks: Reproducibility and Clinical Application. J Neurosci. 2020 Feb 5;40(6):1311-1320. doi: 10.1523/JNEUROSCI.1485-19.2019. Epub 2019 Dec 18. PMID: 31852732; PMCID: PMC7002153.

Isolating naming, semantic retrieval, and familiarity judgment. The three effects are overlaid on five axial slices and a rendered template brain (left and right hemisphere).
Borghesani V, Narvid J, Battistella G, Shwe W, Watson C, Binney RJ, Sturm V, Miller Z, Mandelli ML, Miller B, Gorno-Tempini ML. "Looks familiar, but I do not know who she is": The role of the anterior right temporal lobe in famous face recognition. Cortex. 2019 Jun;115:72-85. doi: 10.1016/j.cortex.2019.01.006. Epub 2019 Jan 23. PMID: 30772608; PMCID: PMC6759326.
fMRI: Functional Language Networks

Spatial distribution of the four task-free networks. Rendered left-hemisphere lateral views of the four ICNs identified through a statistical conjunction of the two groups. Maps were thresholded at the same statistical threshold used to identify the networks for each group separately, binarized and colored using the same color scheme in Figure 1 (blue for the network seeded from the pITG, yellow for the network seeded from the Spt, red for the network seeded from the opIFG, and green for the one seeded from the aMTG). The render at the center of the figure shows that the four networks cover almost the entire left hemisphere with a small degree of overlap.
Battistella G, Borghesani V, Henry M, Shwe W, Lauricella M, Miller Z, Deleon J, Miller BL, Dronkers N, Brambati SM, Seeley WW, Mandelli ML, Gorno-Tempini ML. Task-Free Functional Language Networks: Reproducibility and Clinical Application. J Neurosci. 2020 Feb 5;40(6):1311-1320. doi: 10.1523/JNEUROSCI.1485-19.2019. Epub 2019 Dec 18. PMID: 31852732; PMCID: PMC7002153.
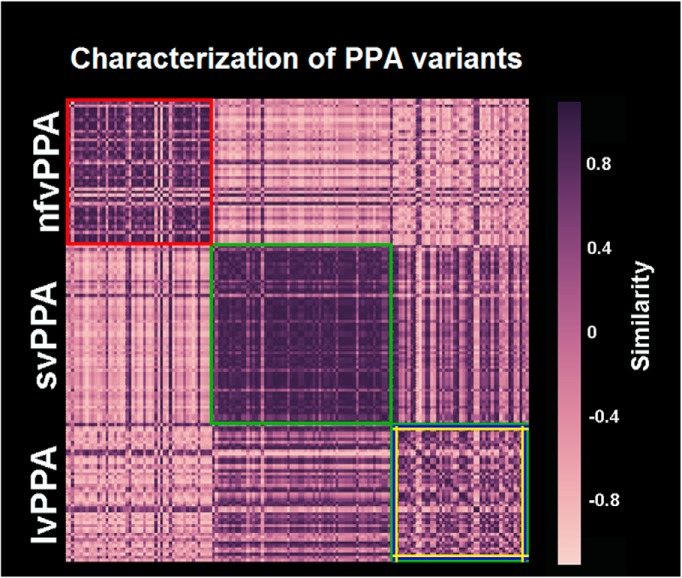
Characterization of PPA variants based on the similarity measure between the four ICNs derived from controls and the atrophy maps of PPA patients. The figure shows the similarity matrix obtained from combining the spatial organization of the four ICNs derived from controls and the atrophy maps of PPA patients. It shows a clear identification of nfvPPA/opIFG ICN (red frame), svPPA/aMTG ICN (green frame), and lvPPA/posterior networks (blue, green, and yellow frames) patients, as visible from the similarity values within each of these variants. The sparse representation of similarity values within the group of lvPPA patients, calls for high intersubject variability of the patients affected by this syndrome.
Battistella G, Borghesani V, Henry M, Shwe W, Lauricella M, Miller Z, Deleon J, Miller BL, Dronkers N, Brambati SM, Seeley WW, Mandelli ML, Gorno-Tempini ML. Task-Free Functional Language Networks: Reproducibility and Clinical Application. J Neurosci. 2020 Feb 5;40(6):1311-1320. doi: 10.1523/JNEUROSCI.1485-19.2019. Epub 2019 Dec 18. PMID: 31852732; PMCID: PMC7002153.
DWI: White Matter Tracts Subserving Language and Speech
Diffusion-Weighted Imaging (DWI) is a neuroimaging technique that measures the random motion of water within brain tissue. By tracking the movement of these water molecules along fiber bundles in the brain (what is referred to as Diffusion Tensor Imaging or DTI), we can reconstruct the axonal (white matter) pathways of the brain to better understand the connections between cortical and subcortical gray matter structure. To facilitate our DTI analyses, we have developed a Cortically Constrained Shape Recognition Pipeline to automatically generate whole-brain tractography of white matter pathways (Jordan, Lauricella, Licata et al., 2021). We have utilized DTI in healthy and clinical populations to investigate differences in white matter pathways and their relation to language and speech. In our research, we found that specific white matter pathways in the left hemisphere of the brain are involved in aspects of speech fluency and are selectively damaged in individuals with non-fluent variant PPA (nfvPPA) compared to individuals with semantic or logopenic variant PPA (Mandelli et al., 2014).

This flow chart shows the steps performed for the automated CCSR segmentation approach. First, the whole-brain tractography is performed in the participant's native diffusion space using High Angular Resolution Diffusion Imaging data, and a FreeSurfer parcellation is performed with the Desikan–Killiany Atlas using the subject's magnetization-prepared rapid acquisition with gradient-echo T1-weighted anatomical MRI. A boundary-based registration is used to bring the FreeSurfer parcellation into the native diffusion space, and the Diffusion Imaging in Python (DIPY) library's whole brain Streamline Linear Registration algorithm is used to align bundle templates with the native diffusion space. Next, the identified a priori inclusion and exclusion regions of interest (ROIs) are used to select the intermediate set of streamlines. Finally, shape recognition is performed using DIPY's RecoBundles to filter out the streamlines that do not match the shape of the tract based on predefined 3-dimensional bundle templates and preset parameters.
Jordan KM, Lauricella M, Licata AE, Sacco S, Asteggiano C, Wang C, Sudarsan SP, Watson C, Scheffler AW, Battistella G, Miller ZA, Gorno-Tempini ML, Caverzasi E, Mandelli ML. Cortically constrained shape recognition: Automated white matter tract segmentation validated in the pediatric brain. J Neuroimaging. 2021 Apr 20. doi: 10.1111/jon.12854. Epub ahead of print. PMID: 33878229.

Schematic representation of the speech-production and language tracts. All of the expected tracts are displayed as schematic connections. All of the ROIs are represented as colored spheres in the 3D brain (MNI space).
Mandelli ML, Caverzasi E, Binney RJ, Henry ML, Lobach I, Block N, Amirbekian B, Dronkers N, Miller BL, Henry RG, Gorno-Tempini ML. Frontal white matter tracts sustaining speech production in primary progressive aphasia. J Neurosci. 2014 Jul 16;34(29):9754-67. doi: 10.1523/JNEUROSCI.3464-13.2014. PMID: 25031413; PMCID: PMC4099550.
MEG: Brain Dynamics in PPA
Magnetoencephalography (MEG) is a functional neuroimaging technique that measures the electromagnetic fluctuations at the level of the scalp thought to reflect millisecond-by-millisecond oscillatory brain activity. In collaboration with the UCSF Biomagnetic Imaging Laboratory (BIL), we are able to scan individuals with PPA in order to directly examine dynamic brain activity with high spatial and temporal resolution, compared to other techniques such as fMRI. Utilizing MEG, we have revealed that patients with semantic variant PPA (svPPA) show differences in the spatiotemporal patterns of neural activation during tasks such as irregular word and non-word reading (Borghesani et al., 2020). This work suggests that the neural pathways and cognitive strategies typically engaged during reading aloud are altered in individuals with svPPA relative to healthy adults, potentially signaling a compensatory mechanism by over-recruiting parietal regions of the brain. In another study, we showed that following ATL damage and corresponding semantic loss, svPPA patients categorize items over-recruiting occipital areas of the brain implicated in perceptual processing (Borghesani et al., 2021).

Behavioral data and atrophy distribution in svPPA patients. (A) Percentage of errors in each experimental condition (average and standard error of the mean), for controls (n = 12) and svPPA patients (n = 11). Darker colors represent the average percentage of regularization errors (with irregular words) and lexicalization errors (in pseudowords). (B) Average reaction times (RTs) in each experimental condition, in control subjects (n = 12) and svPPA patients (n = 11). Errors bars represent the standard error of the mean (SEM). (C) Voxel-based morphometry (VBM)-derived atrophy pattern showing significantly reduced grey matter volumes in the anterior temporal lobe for svPPA patients (thresholded at P < 0.001 with FWE correction). (D) Cartoon representation of the dual-route model: the dorsal sublexical/phonological route supports orthography-to-phonology mapping based on language-specific statistical rules, while the ventral lexico-semantic route maps orthographic forms to meaning thus allowing correct reading of irregular words. HC = healthy controls; LH = left hemisphere.
Borghesani V, Hinkley LBN, Ranasinghe KG, Thompson MMC, Shwe W, Mizuiri D, Lauricella M, Europa E, Honma S, Miller Z, Miller B, Vossel K, Henry MML, Houde JF, Gorno-Tempini ML, Nagarajan SS. Taking the sublexical route: brain dynamics of reading in the semantic variant of primary progressive aphasia. Brain. 2020 Aug 1;143(8):2545-2560. doi: 10.1093/brain/awaa212. PMID: 32789455; PMCID: PMC7447517.
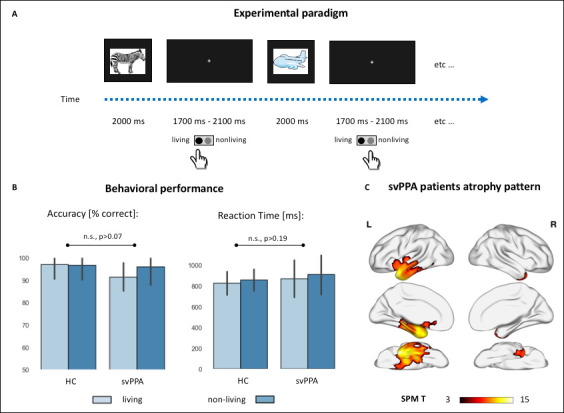
Experimental paradigm, behavioral performance, and cortical atrophy.
(A) Cartoon representation of the experimental setting. Colored drawings were presented for 2 s, with an inter-stimuli interval jittered between 1.7 and 2.1 s. Subjects responded with a button press with their dominant hand. (B) Behavioral performance during the semantic categorization tasks in controls and semantic variant primary progressive aphasia (svPPA) patients, across the two stimuli conditions (living vs. nonliving items). There were no statistically significant effects of diagnosis, category, nor their interaction neither in percentage accuracy (healthy controls [HC]: living: 97.1 ± 6.6, nonliving: 96.8 ± 6.6; svPPA: living: 91.5 ± 6.2, nonliving: 95.9 ± 8.1) nor in reaction times (HC: living: 826.3 ± 112.5, nonliving: 856.9 ± 104.4; svPPA: living: 869.8 ± 179.8, nonliving: 911.1 ± 194.45). (C) Voxel-based morphometry (VBM)-derived atrophy pattern showing significantly reduced gray matter volumes in svPPA patients’ anterior temporal lobes, views from top to bottom: lateral, medial, ventral (thresholded at p<0.05 with family-wise error [FWE] correction, cluster threshold of 100 voxels).
Borghesani V, Dale CL, Lukic S, Hinkley L, Lauricella M, Shwe W, Mizuiri D, Honma S, Miller Z, Miller B, Houde JF, Gorno-Tempini ML, Nagarajan SS. Neural dynamics of semantic categorization in semantic variant of primary progressive aphasia. Elife. 2021 Jun 22;10:e63905. doi: 10.7554/eLife.63905. PMID: 34155973; PMCID: PMC8241439.